Which of the Following Atoms Has the Largest Atomic Radius
In which of the following atoms is the 3s orbital. In determining the radius of an atom we have to consider the number of protons and the how many shells does this atom have.

Chemistry Trends Multiple Choice Diagram Quizlet
Na and Cl.

. D A andor B. Na atoms are larger than Cl atom because as we go left to right in a series size of atoms decreases. Atomic Radius is defined as.
The principal reason that the melting point of N a F is much higher than that of R b B r is that. Element Z is larger than Element X. The element which has the largest atomic radius is Cesium.
As you go down the table more electron shells are added to make the radius larger but as you go to the right the protons attraction increases so the radius actually shrinks. E B andor C. Na has the largest atomic radius.
1s2 2s2 2p6 3s1. Which of the following atoms has the largest atomic radius. Incomplete electronic shells shield the nuclear charge VERY INEFFECTIVELY with the result that atomic.
Serey is waiting for your help. This is because of the increase in nuclear charge which tends to pull the electron closer to the nucleus and reduces the size of the atom. Question 10 1 point Put the following atoms in order from largest to smallest atomic radius largest 1 smallest 3.
In a period table in group from top to bottom atomic radius increases since the nuclear charge decreases decreases down the group due View the full answer Transcribed image text. Hence K has the largest atomic radii. B Element X is closer to the top of the periodic table.
From left to right in a period atomic radius gradually decreases due to increase in nuclear charge. Atoms decrease in size across the period and. So atomic radius decreas.
Barium Ba will have the largest atomic size. Therefore according to the trend Cl has a larger radius because it is lower in the table. Francium has the largest atomic radius since this atom contains the maximum number of shells with the lowest number of protons.
As a result orbital electrons become more closer to nucleus. Along a period the atomic radius decreases due to increase in nuclear charge and down a group the atomic radius or size of atom increases due to increase in a number of atomic shells thus Na will have a largest atomic radius. Which of the following element has highest atomic radius.
This is because of increase in number of electrons and protons in the same shell. Which of the following correctly lists the five atoms in order of increasing size smallest to largest. Hence the correct option is C.
And ii shielding by other electrons. Atomic radius means the distance between the farthest electron and the center of the nucleus. Atomic radii increase toward the bottom left corner of the periodic table with Francium having the largest atomic radius.
Which of the following elements has the largest atomic size. The element which has the largest atomic radius is Cesium. In periodic table 3rd period will have larger atomic radius than the second period.
Helium 3A 13 4A 14 5A 15 6A 16. A Element Z is further to the left side of the periodic table. K has the largest atomic radii because atomic radii decrease from left to right along a period.
The size of an atom is reasonably the radius of its valence electron s. The element potassium K present in group 1 has the largest atomic radius. Which one of the following elements has the largest atomic radius.
The melting point of R b B r is 6 8 2 o C while that of N a F is 9 8 8 o C. C Element Z and X are probably in the same group. Answer 1 of 4.
Elements Z and X are compared. Na Z 11. What group has smallest atomic radius.
March 29 2022. As the nuclear charge increases the attractive force between nucleus and electrons increases. This radius is i a function of Zthe atomic number.
Based on this you could say. Has the largest radius. Atomic Radius is defined as.
Atomic size INCREASES down a Group a column of the Periodic Table. Belong to the 3rd period. Which among the following element has largest atomic size.
Belongs to the 2nd period. Again Cl lies to the right and Na lies to the left of the periodic table. CL has a larger Atomic Radius.

Solved 25 Select The Period Element That Has Four Valence Electrons A Zr B Hf Pb E Sn Which Of The Following Atoms Has The Largest Atomic Radius 2 F B Na Mg
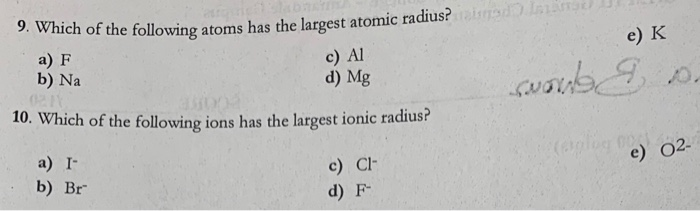
Solved Which Of The Following Atoms Has The Largest Atomic Chegg Com
Atomic Radius Trend Periodic Table Chemtalk

Solved Which Of The Following Elements Has The Largest Chegg Com

Comments
Post a Comment